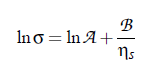2018-(II)-Surface Tension in Critical Mixtures
Article Index
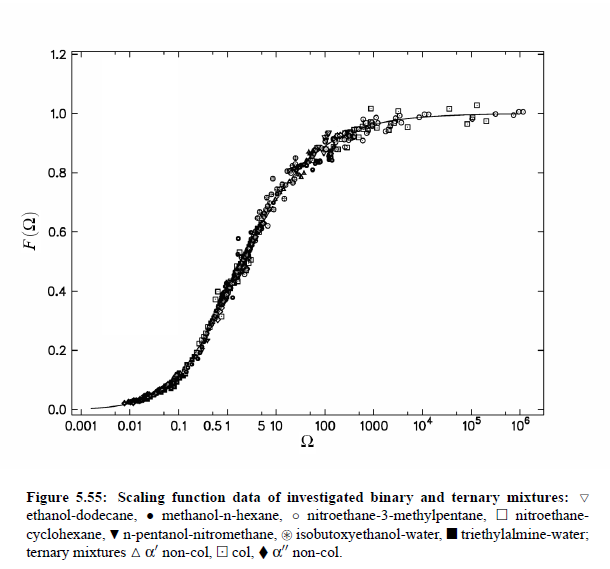
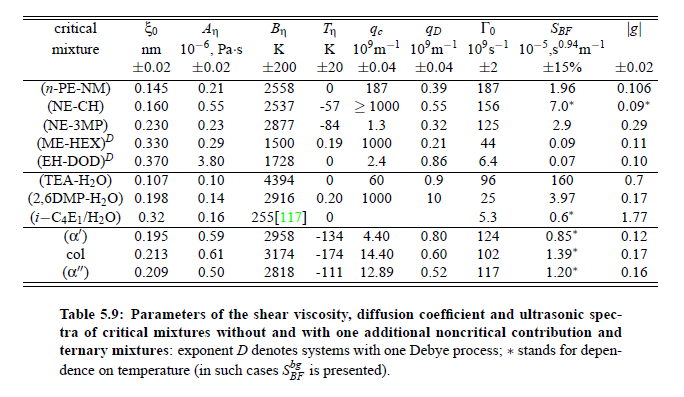
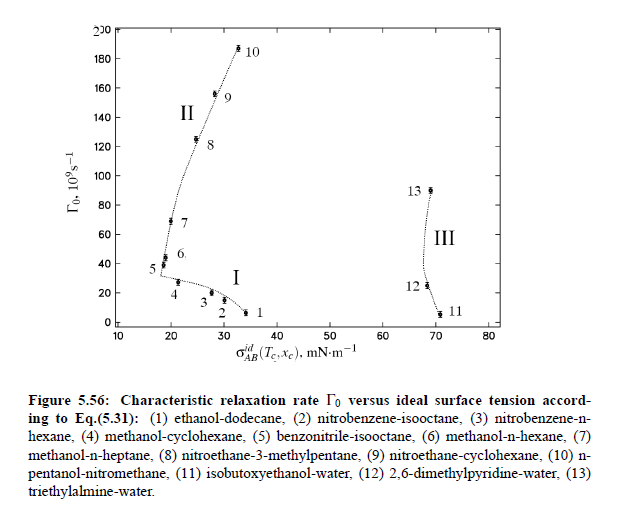
Parameters which have been obtained from ultrasonic spectrometry, dynamic light scattering and shear viscosity are presented in Table (5.9). It is a fascinating aspect of the dynamic scaling theory of ultrasonic attenuation that, due to scaling of frequency data of different critical mixtures fall on one scaling function. However, the most curious specific system parameter is the characteristic relaxation rate amplitude Γ_0, which according to Bhattacharjee-Ferrell theory, corresponds with the mutual diffusion coefficient D and the fluctuation correlation length ξ. In Table (5.9) parameters Γ_0 and ξ_0 are listed for various binary mixtures with critical demixing point. The isobutoxyethanol-water system exhibits by far the smallest amplitude Γ_0 in the relaxation rate of order parameter fluctuations. In comparison, with the system n-pentanol-nitromethane, Γ_0 is 35 times larger. Assuming, that the life time of fluctuations τξ = Γ_0^−1, as inverse characteristic relaxation rate reflects intermolecular properties as well the geometry of considered components the strong variation of Γ_0 of various liquids can be understood. In addition, due to the Coulombic interactions, relaxation from a local nonequilibrium distribution of electrical charges into thermal equilibrium will involve extensive redistribution of ions in ionic solutions and may, therefore, proceed with a smaller relaxation rate than a molecular liquid mixture at the same reduced temperature. A quantity, which may be taken to summarize the above mentioned molecular properties, is the surface tension σ. If considering critical fluctuations, reflected by the fluctuation relaxation rate Γ_0 , to depend on the surface tension, a correlation between both quantities should exist.
Based on this idea, Khabibullaev and Mirzaev found a correlation between sound attenuation and surface tension. One of the most important parameters in the determination of the characteristic relaxation rate is the viscosity. The first relation between surface tension and viscosity, has been presented by Pelofsky [149] as a relation between these two thermophysical properties:
where A and B are constants, σ is the surface tension, and ηs the viscosity. According to this equation, this empirical expression can be applied for pure and mixed components. Several fluids were shown to follow these relations: n-alkanes, benzene, toluene, xylenes, phenol and other aromatics, n-alcohols in the range, water and some aqueous solutions.
- Prev
- Next >>